Sanders Lab – Dept Chemistry – U Saskatchewan
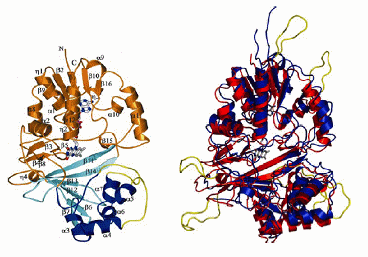
We are studying the role of an enzyme, UDP-Galactopyranose mutase, in a simple eukaryotic model system. Mutase catalyzes the interconversion of 5- and 6-membered forms of galactose prior to its incorporation into the cell wall or the extracellular matrix, depending on the organism. The structure of mutase was first determined for prokaryotes including E. coli (below, left). Genomics projects have revealed similar sequences in eukaryotes including Leishmania major (below, right). We will be characterizing the Aspergillus nidulans mutase homologue.
Dr. Karin von Straaten (kev138.mail@usask.ca.undefined)
The images:
-
on the left, mutase from Eschericia coli (Sanders et al 2001 Nature Structural Biology 8: 858-63), with three domains shown in different colours.; and
-
on the right, proposed structure of mutase from Leishmania major shown in blue, threaded onto the E. coli structure shown in red using Modeller.